Without a doubt, mycotoxins can be harmful to our health, especially if we are inundated with mold within closed home or office spaces. When it comes to food sources, however, questions arise as to whether certain foods should be eliminated to reduce exposure to mold, and if there are health implications to consuming foods with mycotoxins. Dietary restrictions should not be implemented frivolously, especially for individuals who may already be overwhelmed with nutrition interventions and eliminations. Documentation within the literature and through clinical practice has shown the demise of patients with regards to their nutritional status, microbiota function, and quality of life, due to restrictive diets. The decision to remove certain foods should be evidence-based, and a diet plan should present a clear benefit to the patient. Thus, the goal of this article is to provide substantiation for the idea that eliminating certain foods can reduce mycotoxin exposure in many situations. We begin by looking at the most relevant mycotoxins in our food supply, and then examining the data regarding mycotoxins frequently found in food, along with assessing the possible role of food mycotoxins in the development of mycotoxicosis.
Before we delve into a literature review of mycotoxins in foods, let us briefly discuss why these mycotoxins are pertinent to our health. Included are the most relevant of the common mycotoxins, along with their potential impact on biological systems.
Aflatoxin
Toxicity and carcinogenicity have been found in both human and other animal populations. High acute exposure can lead to death, while chronic exposure can cause immune suppression and even cancer. The liver is the main organ affected in several animal classes (fish, birds, rodents, and primates).
Ochratoxin A (OTA)
Nephrotoxic to all animal species, and in most animals, a hepatotoxic , immune suppressant, a teratogen, and a carcinogen. Primarily, its biggest impact seems to be inhibition of the enzyme involved in synthesizing the phenylalanine-tRNA complex, as well as, affecting ATP production in the mitochondria, and lipid stimulating peroxidation.
Trichothecenes
(ex: Deoxynivalenol, or DON): Cause various symptoms, from alimentary hemorrhaging and vomiting after high consumption, to dermatitis from smaller exposures. In several vertebrate organisms, studies have shown an impact on almost every major system, mostly associated with gastrointestinal, dermatological, and neurological symptoms.
Patulin
Very toxic in high concentrations, but evidence of poisoning in a natural environment is still inconclusive.
Fumonisins
A wide range of effects on various mammals have been observed, centering mainly on interference with sphingolipid metabolism. Studies have demonstrated leukoencephalomalacia in equines and rabbits, pulmonary edema and hydrothorax in swine, and hepatoxic and carcinogenic effects in rats.
Zearalenone
Effects mainly involve hormone disruption. This mycotoxin’s structure resembles 17Beta- estradiol, as expected, it impacts estrogen levels, and ultimately fertility.
Citrinin
Nephrotoxic to all animal species, but the threshold of acute toxicity is highly variable among species. Simultaneous exposure to both Citrinin and OTA can depress RNA synthesis in kidneys.
A glance back in history reminds us of the detrimental impact that mold and mycotoxins has had on our food supply: ergot poisoning from contaminated rye (“St. Anthony’s fire”, 1100 BCE-1800 CE); cardiac beriberi associated with Penicillium molds in rice (“yellow rice toxins”, 2006 in Brazil; 1937, 1948, and 1951 in Japan); and alimentary toxic aleukia associated with Fusarium molds on overwintered wheat, millet, and barley (USSR, World War II era). In 1974, acute Aflatoxin poisoning from moldy corn in Western India caused 100 deaths, and evidence suggests that the adults may have eaten 2 to 6 mg of aflatoxin in a single day. This event led to the calculation of 10 to 20 mg of aflatoxins as the acute lethal dose for adults. In 2004, Aflatoxin poisoning fatal to 125 people occurred in Kenya from contaminated corn (estimated contamination rate ranged from >20 to >1,000 µg/kg). Continuing investigation of mycotoxin-contaminated food suggests that it is still quite common, but to a much lesser extent, at least in developed countries. The UN Food and Agriculture Organization (FAO) assessment indicates that 25% of global fodder crops are contaminated with mycotoxins. Shiratori N, et al. investigated penicillium contamination on rice in Thailand, and found that only 1 of 10 samples contained Aflatoxin B mycotoxin (5.9 μg/kg), although 7 of 10 samples did have trace amounts of Penicillium species isolates. A study conducted on Green Coffee Beans from Brazil found that 91.7% of the 60 samples were contaminated with mold isolates (mainly aspergillus), but only 33% had OTA (0.2- 7.3 μg/kg). Keep in mind, this is below the acceptable limit proposed by the European Union (<8 μg/kg), and below the US limit of <20ppb. So wait, it’s being monitored? Yes! To some extent.
Mold and mycotoxin contamination prevention have become part of the food and agricultural industry, which continues to work on monitoring and reducing it, in part by developing with regulatory standards. Aspergillus, Penicillium, and Fusarium are the most common molds affecting our food supply. Guidelines are in place for the most prevalent mycotoxins and the most common contaminated foods in order to prevent toxic levels.
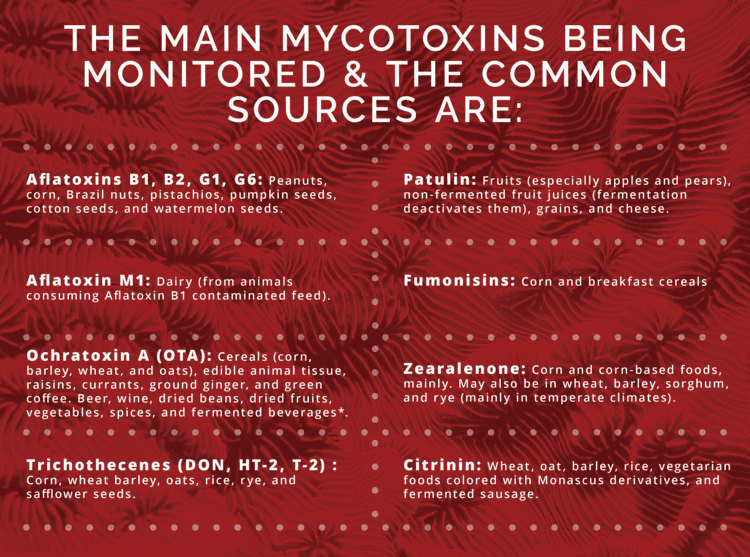
Allowance levels differ based on the mycotoxin, the crop, and its destination. Below is a table that provides some information for the mycotoxin allowance in the United States. Please note, that this list is not exhaustive, but serves as a tool for insight into allowance levels. As noted previously, the European Union is also monitoring, and may differ on amount allowance. For the scope of this article, US standards will be the focus.
| MYCOTOXIN | FOOD | ALLOWANCE | |
|---|---|---|---|
| Aflatoxin (Aspergillus flavus and A. parasiticus) | Action level/limits | ||
| Corn and peanuts intended for beef cattle | 300 ppb | ||
| Corn and peanut products intended for swine greater than 100 lbs | 200 ppb | ||
| Cottonseed meal intended for beef, cattle, swine, or poultry (regardless of age, breeding status or finishing status) | 300 ppb | ||
| Corn and peanuts intended for breeding livestock | 100 ppb | ||
| Corn, peanut products, and other animal feeds and feed ingredients (excluding cottonseed meal), intended for immature animals | 20 ppb | ||
| Brazil nuts | 20 ppb | ||
| Foods for human consumption | 20 ppb (Europe is 0.1-2 µg/kg) | ||
| Peanut and peanut products | 20 ppb | ||
| Pistachios | 20 ppb | ||
| Aflatoxin M1 | Milk | 0.5 ppb | |
| Ochratoxin A (Aspergillus and Penicillium) | Rye flour, wheat flour, some corn products, barley (cereals), oats, both whole and cereals, dried beans, cornmeal, raisins, coffee, soy flour, soy based baby foods, and ground ginger | Less than 20 ppb does not require reporting, more tan 20 ppb needs to be sent off for evaluation by the FDA. | |
| Deoxynivalenol DON (Fusarium) | Advisory Limits | ||
| Corn and grains intended for immature animals and for dairy animals | 1 ppm | ||
| Corn and other grains intended for breast feeding beef cattle, breeding swine, or mature poultry | 5 ppm | ||
| Corn and other grains intended for finishing swine of greater than or equal to 100 lbs | 10 ppm | ||
| Fumonisins | Action level/limits | ||
| Foods for human consumption | 2 – 4 mg/kg * (Max allowed set by FDA) | ||
| Foods for animal consumption | 1-100 ppm, depending on animals | ||
| Patulin (Penicillium, Aspergillus, and Byssochylamys) | Apple juice | 50 ppb | Action level/limits |
**1ppb = 1µg/kg
There is a difference between an action level and an advisory limit. If the mold level in a food is at or above the Action Level, the FDA will take legal action to remove the product from the market. If a food mold level meets an advisory limit, governmental recommendation indicates that it may be a potential health hazard.
Only corn and peanuts are tested for aflatoxin, per the FDA. They state that “nuts (almonds, Brazil nuts, macadamias, pecans, pistachios, walnuts, and hazelnuts) are susceptible to aflatoxin contamination, but samples of these nuts have been largely in compliance [less than 1% ] for several years. Per the FDA, OTA is being monitored and measured, but a limit for ochratoxin in the food supply has not yet been established. A sample is reported if the ochratoxin result is above a certain threshold (>20 ppb).
Statements from the US FDA conflict with the scientific literature; the FDA notes limited data on OTA while the literature contains a significant amount of data on ochratoxin effects, especially nephrotoxicity. The International Agency for Research on Cancer (WHO) has rated OTA as a possible human carcinogen (Category 2B). The European Union Scientific Committee recommends the OTA be reduced to <5 ng/kg, but the allowance varies by country and by crop. Because OTA appears to be less heat-stable than Aflatoxin or DON (which is extremely stable during food processing), the FDA considers it less of a threat for human consumption. A 2016 study out of the University of Idaho tested OTA levels at different temperatures, times, and pH conditions. The largest degradation of OTA (90%) was at 200°C (almost 400 °F), except for samples at a pH of 4. The US does not have contamination standards on zearalenone levels, but the EU requires < 75-350 µg/kg, depending upon the food category. Even a level of 1ppm has been shown to lead to hyperestrogenic syndromes in swine. The FDA categorizes it in a similar manner to OTA contaminated samples, and reports them to the Center of Veterinarian Medicine to determine if regulatory action is required. Citrinin is not monitored, although it is known to be commonly associated with human food.
Organic foods deserve a special mention when considering mycotoxin avoidance. Quite a few studies support the claim that organic foods do tend to have more mycotoxin contamination, mainly due to use of less potent fungicides. In a 1995 study, conventional and alternatively grown rye and wheat were found to have to have a significant difference of DON mycotoxin levels. Conventional rye had an average of 160 µg/kg, while the organically grown rye had an average of 427 µg/kg. Zearalenone in wheat was also investigated and found to have an average of 6 µg/kg in conventional, compared to 24 µg/kg in the alternatively grown crops. A French study comparing organic and conventionally grown wheat generated similar findings: the organic version had an average of 106 µg/kg of DON, whereas conventional wheat had only 55 µg/kg. Apples have also been investigated, with similar results; conventionally grown apples had a median of 35.85 µg/kg of patulin mycotoxins, while the organic apples had a median of 211 µg/kg. This presents a significant barrier to reducing mycotoxins in the diet, since organic foods are a large part of nutrition therapy in Integrative Medicine.
The scientific community is continuing to discover the intricacies of mycotoxins interactions with organisms, and their resulting impact on the food supply and on human health. To date, 300 mycotoxins (and counting) have been identified, although 100,000 mold species (still counting) have been discovered, requiring effort to understand how they affect us. Along with monitoring at least the highly susceptible food sources, considerable resources are expended to hinder food contamination. Prevention seems to be the key aspect to reducing mycotoxins and mold in food. New and fresher solutions to lowering mycotoxins in food include earlier detection of fungus (i.e., biosensors), breeding host plants for greater fungal resistance or using genetic engineering to add antifungal genes, and even targeting genes that regulate mycotoxin synthesis in fungal species, along with the standard approach of applying fungicides at various stages of growth, harvesting, transportation, and sale of foodstuffs.
In summary, we have discussed the most frequent sources of mycotoxin contamination, the most susceptible foods, and strategies in place for monitoring and preventing mycotoxins from entering our food supply, now, how do we apply this information to clinical practice?
It appears that some exposure to mycotoxins is almost inevitable, and a truly “low mycotoxin” or “low mold” diet would leave one with very few food options. Before embarking on a project to eliminate mold exposure from food, it is imperative to take in the entire clinical picture as individual reactions vary widely. Ranges for potential exposure have been developed based on available data and clinical observation. However, it is necessary to consider a person’s; age, body composition, nutrient status, comorbidities, allergen sensitivities, genetics, current toxin burden, the effect of a combination of different mycotoxins, etc., etc., as all have shown to impact the severity of symptoms and their progression. A look into the literature suggests that some diseases may be associated with food mycotoxins, but the evidence is incredibly difficult to assess over a lifetime. For example, epidemiological studies have shown correlations with increased incidence of hepatic carcinoma in populations with higher dietary aflatoxin exposure in addition to diagnosis of hepatitis B. An overall strategy, particularly for high-risk populations may involve reducing or removing foods, likely to have fungal contamination, with the clear understanding that individual response to mycotoxin exposure is not predictable.
Dietary restrictions to avoid mold exposure should be decided between provider and patient, with the assumption that it is probably not possible to be completely free of mycotoxins in food. The goal should be to reduce the load as much as is practical for the individual. We hope this evidence-based discussion will help in designing individual versions suitability of the “mold diet”.
References
1. Ehling, G., Cockburn, A., Snowdon, P., and Buchhaus, H. (1997) The significance of the Fusarium toxin deoxynivalenol (DON) for human and animal health. Cereal Research Communications, 25: 443-447.2. Khera KS, Arnold DL, Whalen C, Angers G, Scott PM. Vomitoxin (4-deoxynivalenol): effects on reproduction of mice and rats. Toxicol Appl Pharmacol. 1984 Jul;74(3):345-56. doi: 10.1016/0041-008x(84)90288-6. PMID: 6740683Kuiper-Goodman T. Mycotoxins: Risk assessment and legislation. Toxicol Lett. 1995 Dec; 82-83:853-9. doi: 10.1016/0378-4274(95)03599-0. PMID: 8597153Vanhoutte I, Audenaert K, De Gelder L. Biodegradation of Mycotoxins: Tales from Known and Unexplored Worlds. Front Microbiol. 2016; 7:561, Published 2016 Apr 25. doi:10.3389/fmicb.2016.00561Horgan, J. (2020, July 17). St. Anthony's Fire. Ancient History Encyclopedia. Retrieved SOURCEShiratori N, Kobayashi N, Tulayakul P, et al. Occurrence of Penicillium brocae and Penicillium citreonigrum, which Produce a Mutagenic Metabolite and a Mycotoxin Citreoviridin, Respectively, in Selected Commercially Available Rice Grains in Thailand. Toxins (Basel). 2017;9(6):194. Published 2017 Jun 15. doi:10.3390/toxins9060194Martins ML, Martins HM, Gimeno A. Incidence of microflora and of ochratoxin A in green coffee beans (Coffea arabica). Food Addit Contam. 2003 Dec;20(12):1127-31. doi: 10.1080/02652030310001620405. PMID: 14726276Wan, J, Chen, B, Rao, J. Compr Rev Food Sci Food Saf. 2020; 1– 27.U.S. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health, National Institute of Environmental Health Sciences. Environmental Health Perspectives. Volume 107, 10/1999. Pg A515Haschek WM, Voss, KA, Beasley VR. Handbook of Toxicologic Pathology (Second Edition), 2002. SOURCEBennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16(3):497-516. doi:10.1128/cmr.16.3.497-516.2003Riches, E. Organic Food- The Hazard of Mycotoxins. CABI. 2003. SOURCEMarx, H., Gedek, B. and Kallarczik, B. 1995. Vergleichende untersuchungen zum mykotoxikologischen status von ökologisch und konventionell angebautem getreide. Zeitschrift für Lebensmitteluntersuchung und –forschung. 201: 83-86.Bhatnagar, D., Payne, G.A., Cleveland, T.E., Robens, J.F. Mycotoxins: Current issues in U.S.A. Proceedings of the 2nd Second World Mycotoxin Forum, February 17-18, 2003, Noordwijk, The Netherlands.Guidance for Industry: Action Levels for Poisonous or Deleterious Substances in Human Food and Animal Feed. 2000. Food and Drug Administration. SOURCEBrown, R. L., D. Bhatnagar, T. E. Cleveland, and J. W. Cary. 1998. Recent advances in preharvest prevention of mycotoxin contamination, p. 351-379. In K. K. Sinha and D. Bhatnagar, (ed.), Mycotoxins in agriculture and food safety. Marcel Dekker, Inc., New York, N.YFood and Drug Administration. CVM Annual Report on Mycotoxins in Animal Food Report for Fiscal Year 2016. SOURCEFood and Drug Administration Office of Regulatory Affairs ORA Laboratory Manuel Volume IV Section 7. Mycotoxin Analysis. 2020. SOURCESudhakar P, AswaniKumar YVV, Bodaiah B, Mangamu UK, Vijayalakshmi M and Renuka RM (2016) Mycotoxin Strategies: Impact on Global Health and Wealth. Pharm Anal Acta 7: 498. doi:10.4172/2153-2435.1000498Mateo, R., A. et.al, 2007. Intl. J. Food Microbiol. 119 (1–2): 79–83. 11. Yin, YN et al., 2008, J Zhejiang Univ. Sci. B 9 (10): 787–92