CLOSTRIDIA DETECTION BY ORGANIC ACIDS TEST
WHY THE GPL ORGANIC ACIDS TEST IS SUPERIOR TO STOOL TESTS FOR DETECTING CLOSTRIDIA
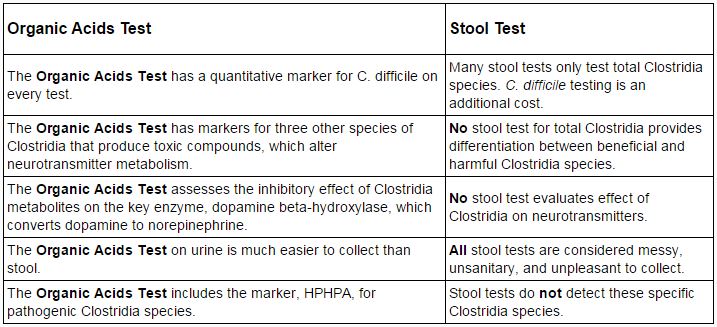
Continued research at The Great Plains Laboratory (GPL) has resulted in new information on several Clostridia bacteria markers that are now included in the urine organic acids test. These markers include 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), 4-cresol, 4-hydroxyphenylacetic acid, and 3-indoleacetic acid. With this new information, The Great Plains Laboratory urine organic acids test now provides information not available in common stool tests for Clostridia bacteria that are involved in gastrointestinal diseases and neuropsychiatric disorders.
BENEFITS OF ORGANIC ACIDS TESTING FOR CLOSTRIDIA
TOXIC METABOLITES OF CLOSTRIDIA BACTERIA
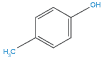
HPHPA (3-(3-hydroxyphenyl)-3-hydroxypropionic acid)
The primary Clostridia species which produce HPHPA include C. botulinum, C. sporogenes, and C. caloritolerans. C. botulinum is a Gram-positive, rod-shaped, anaerobic, spore-forming, motile bacterium with the ability to produce the neurotoxin botulinum. Symptoms of botulism include weakness, impaired vision, fatigue, and difficulty with speech. These may then be followed by weakness of the arms, chest muscles and legs. In food borne botulism, symptoms generally begin 18 to 36 hours after eating a contaminated food, but they can occur as early as 6 hours or as late as 10 days. C. sporogenes is virtually identical to C. botulinumexcept it is lacking the gene for the botulinum neurotoxin. Like C. botulinum, it is an anaerobic Gram-positive, rod-shaped bacterium that produces oval, subterminal endospores and is commonly found in soil. C. caloritolerans is named after the fact that it is extremely heat (calor) resistant (tolerans). It can survive at the water temperature boiling point for 8 hours; its extreme resistance to heat may allow transmission even in well-cooked food.

4-Hydroxyphenylacetic acid
High 4-hydroxyphenylacetic acid may be associated with small intestinal bacteria overgrowth (SIBO) due to its production by C. difficile, C. stricklandii, C. lituseburense, C. subterminale, C. putrefaciens, and C. propionicum. C. difficile can be distinguished from the other species by its production of 4-cresol. No other Clostridia species produce 4-cresol. Elevated values of 4-hydroxyphenylacetic acid are common in celiac disease and cystic fibrosis and have been reported as elevated in jejuna web, transient lactose intolerance, Giardia infection, ileal resection, ileo-colic intersusseception, septicemia, and projectile vomiting. Elevations of 4-hydroxyphenylacetic acid in celiac disease and cystic fibrosis are common enough to suggest that these Clostridia bacteria may play a role in these illnesses.

3-Indoleacetic acid
High 3- indoleacetic acid in urine is a tryptophan byproduct of C. stricklandii, C. lituseburense, C. subterminale, and C. putrefaciens. No information is available on the pathogenicity of these species producing indoleacetic acid. However, very high amounts of this metabolite derived from tryptophan might indicate a depletion of tryptophan needed for other physiological functions.

4-Cresol
4-Cresol is predominantly produced by C. difficile, a pathogenic bacteria, that is one of the most common pathogens spread in hospitals and nursing homes. Toxin-producing strains of C. difficile can cause illness ranging from mild or moderate diarrhea to pseudomembranous colitis, which can lead to toxic dilatation of the colon (megacolon), sepsis, and death. 4-cresol (para-cresol) has been used as a specific marker for C. difficile. It is classified as a toxic agent and can cause rapid circulatory collapse and death in humans. Intestinal production of 4-cresol may be responsible for growth-suppressing effect in animals. Signs of acute toxicity in animals typically include hypoactivity, salivation, tremors and convulsions. High amounts of 4-cresol have been found in the urine of patients with autism.
HOW CLOSTRIDIA METABOLITES INHIBIT KEY NEUROTRANSMITTERS IN THE BRAIN AND PERIPHERAL NERVOUS SYSTEM
The combined metabolic pathway for production of human neurotransmitters in the brain, adrenal gland, and sympathetic nervous system is outlined in Figure 1 below. The key starting material for both human and Clostridia pathways is the amino acid phenylalanine.
In humans, phenylalanine and/or tyrosine from dietary proteins or amino acid supplements are absorbed into blood from the intestinal tract where these amino acids cross the blood-brain barrier and enter the brain. Phenylalanine in the brain is converted to tyrosine by phenylalanine hydroxylase. The ring of tyrosine is then hydroxylated to dihydroxyphenylalanine (DOPA) by tyrosine hydroxylase. DOPA is then converted to dopamine by DOPA decarboxylase which requires a vitamin B6 cofactor. The fate of further dopamine metabolism depends on the neuron type. In dopamine-secreting neurons, dopamine is the final product. In these neurons, dopamine is metabolized into homovanillic acid which can be measured in the urine organic acid test. In norepinephrine-containing brain neurons, neurons in the peripheral central nervous system, and in the adrenal gland, dopamine is converted to norepinephrine by dopamine-beta-hydroxylase. Dopamine-beta-hydroxylase requires ascorbic acid and copper as cofactors. In the adrenal gland, norepinephrine is further converted to epinephrine. Both epinephrine and norepinephrine may then be metabolized into vanillylmandelic acid (VMA).
In the species of Clostridia bacteria mentioned at the beginning of the article, phenylalanine is converted to HPHPA by a pathway that requires both human and bacterial enzymes. If Clostridia difficile is present, tyrosine is largely converted to 4-cresol. These byproducts are then absorbed into the body through the intestinal tract where they have the ability to inhibit dopamine-beta-hydroxylase. These byproducts covalently bind to the enzyme active site, irreversibly inhibiting conversion of dopamine to norepinephrine.
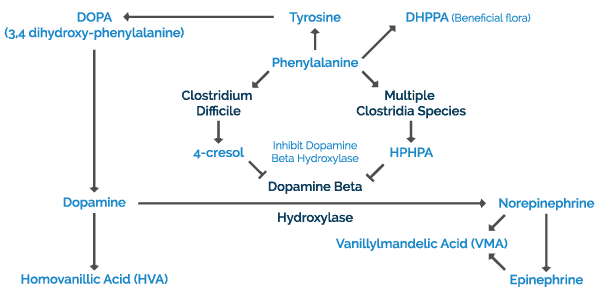
INTERACTION BETWEEN CLOSTRIDIA MARKERS AND NEUROTRANSMITTERS
The Great Plains Laboratory Organic Acids Test is very sensitive and can differentiate between harmful and beneficial bacteria, which is is unique among tests for Clostridia. It is also the only organic acids test available that measures HPHPA, one of the primary toxic metabolites of Clostridia. In the sample results below, the patient has a high level of HPHPA, but a low level of 4-Cresol, the main marker for C. difficile. Other tests that only measure 4-Cresol, and not HPHPA, would have missed this harmful bacterial overgrowth.
Another benefit to The Great Plains Laboratory Organic Acids Test is that it evaluates Clostridia metabolites that can inhibit metabolism of important neurotransmitters. As shown in the sample results below, the test measures Homovanillic Acid (HVA), a metabolite of dopamine, as well as vanillylmandelic acid (VMA), a metabolite of norepinephrine and epinephrine. Clostridia bacteria can produce toxins that may inhibit the conversion of dopamine to norepinephrine. This can lead to a buildup of dopamine and a disruption in the dopamine to norepinephrine ratios. By assessing these metabolites, the Organic Acids Test is able to better determine the possible underlying cause of many different conditions.
MORE ABOUT THE ORGANIC ACIDS TEST
The Organic Acids Test (OAT) offers a comprehensive snapshot of many crucial metabolic processes. Besides offering a thorough assessment of intestinal yeast and bacteria, it also provides information about Krebs cycle functionality, neurotransmitter levels, nutritional markers, glutathione status, and oxalate metabolism. The test offers a total of 74 urinary metabolite markers that can reveal underlying causes of chronic health conditions including mental health disorders, autoimmune diseases, ADHD, autism, and other illnesses. This reliable test detects the overgrowth of yeast and toxigenic Clostridia bacteria, commonly missed by conventional culture methods. These organisms and their metabolites can produce or magnify symptoms of many disorders. Once abnormalities are detected, a variety of options are available for treatment. Treatments include antifungal or antibacterial products, probiotic supplementation, vitamins, antioxidants and dietary modification. Patients and physicians have reported significant improvement after treatment, including decreased fatigue, regular bowel movements, increased energy and alertness, increased concentration, improved verbal skills, less hyperactivity, better sleep patterns, and less abdominal pain.
POSSIBLE TREATMENTS FOR CLOSTRIDIA
The oral antibiotics metronidazole and vancomycin are very effective in treating the growing vegetative cells of Clostridia, but are ineffective against spores. Pulsing protocols that include a waiting period without antibiotic treatment allow antibiotic resistant spores to revert to their antibiotic-susceptible vegetative forms. Use of these pulsing protocols markedly reduces the recurrence rate for Clostridia. Probiotics have been effective in conjunction with high doses of vancomycin or metronidazole. Several strains of Lactobacilli have proven effective for recurring Clostridium difficile infections, including Lactobacillus rhamnosus GG (the main probiotic bacteria in Culturelle®), as well as Saccharomyces boulardii, a beneficial strain of yeast.
REFERENCES Beatty, H. Botulism. In: Harrison's Principles of Internal Medicine, 10th edition, ed. R. Petersdorf, et al. McGraw Hill. New York. 1983. Pages 1009-1013. Meyer, K.F. and Lang, O.W. A highly heat-resistant sporulating anaerobic bacterium: Clostridium caloritolerans, N. SP. The Journal of Infectious Diseases Vol. 39, No. 4 (Oct., 1926), pp. 321-327 Chalmers, R.A., Valman. H.B., and Liberman, M.M., Measurement of 4-hydroxyphenylacetic aciduria as a screening test for small-bowel disease. Clin Chem 25:1791, 1979 Carrico, R.M. Association for Professionals in Infection Control and Epidemiology (APIC) Implementation Guide to Preventing Clostridium difficile Infections http://apic.org/Resource_/EliminationGuideForm/59397fc6-3f90-43d1-9325-e8be75d86888/File/2013CDiffFinal.pdf (accessed Oct 30,2014) Sivsammye, G. and Sims, H.V. Presumptive identification of Clostridium difficile by detection of p-cresol (4-cresol) in prepared peptone yeast glucose broth supplemented with p-hydroxyphenylacetic acid. J Clin Microbiol. Aug 1990; 28(8): 1851–1853. Phua, T.J., Rogers, T.R., and Pallett, A.P. Prospective study of Clostridium difficile colonization and paracresol detection in the stools of babies on a special care unit. J. Hyg., Camb. (1984). 93. 17-25 17 Yokoyama, M. T., Tabori, C., Miller, E. R. and Hogberg, M. G. (1982). The effects of antibiotics in the weanling pig diet on growth and the excretion of volatile phenolic and aromatic bacterial metabolites. The American Journal of Clinical Nutrition 35, 1417-1424. Persico, A.M. and Napolioni, V. Urinary p-cresol (4-cresol) in autism spectrum disorder. Neurotoxicology and Teratology 36 (2012) 82–90 Wells, J.M. and Allison, C. Molecular genetics of intestinal anaerobes. In: Human Colonic Bacteria. Role in Nutrition, Physiology, and Pathology. Gibson and MacFarlane, ed. CRC Press. Ann Arbor. 1995. Page28 Conway, P. Microbial ecology of the human large intestine. In: Human Colonic Bacteria. Role in Nutrition, Physiology, and Pathology. Gibson and MacFarlane, ed. CRC Press. Ann Arbor. 1995. Pages 1-24